Heat Capacity of Calorimeter
195 Degrees C Ending temperature 56 Degrees C Mass of calorimeter water after passing steam 0151Kg Mass of the condensed steam 0006Kg Heat gained by the water. Temperature changedT - 2686 oC to 2943 oC.

Specific Heat Capacity Physics Lessons Science Teaching Resources Science Facts
Q is the heat absorbed or released by a material J.

. The calorimeter consisted of two styrofoam coffee cups one inside the other. Thermochemistry determine the heat exchanged at constant pressure q m c T. Heat Capacity or Thermal Capacity.
Figure 1 shows the block diagram of Heat Flux DSC as an example. C g specific heat capacity of solutions 418 JC-1 g-1 assumed q heat liberated during neutralisation reaction. The temperature of the calorimeter rises from 250 to 310 K.
Anyone with access to a kitchen can do a form of this experiment and become a thermal physicist. And the heat captured by the calorimeter Q water is 150 g 0001 Calg C 133C or 20 Cal. Say in a calorimeter a fixed amount of fuel is burned.
Calculating the limiting reactant the change in enthalpy of the reaction H rxn can be determined since the reaction was conducted under conditions of constant pressure H rxn q rxn moles of limiting reactant. Why did you select this example. 3234 Thermal capacity of calorimeter and water.
A bomb calorimeter or a constant volume calorimeter is a device often used to determine the heat A. Heat loss by the fuel is equal to the heat gained by the water. HClaq NaOHaq -- NaClaq H 2 Ol Energy.
Thermal capacity of water. 131 Specific heat capacity. Heat capacity c 893 kJk.
If you have it in Jkg C then you need the mass of the substance in kilograms. Check the units for consistency and convert if necessary. In this required practical activity it is important to.
References Theory of Heat Maxwell James Clerk page 57-67 Westport Conn Greenwood Press 1970. Block diagram of Heat Flux DSC. C is the specific heat of a material JgK.
For example the lower specific heat capacity of fat compared to other soft tissue indicates that fat requires. It uses the time-tested Parr 1108 style oxygen bomb and oval bucket in a compact calorimeter producing reliable results with good repeatability but differing from the 6400 Model in that the bomb and bucket. The specific heat capacity c Jkg K of tissue describes how much energy is required to change the temperature of 1 kg of tissue by 1 K 1C.
Model 6200 is a microprocessor controlled isoperibol oxygen bomb calorimeter which is widely used for both routine and occasional calorific tests. Heat capacity ratio of heat absorbed by a material to the temperature change. C is the specific heat capacity of water which is 1 calg C 1 calorie per gram per degree Celsius.
Such measurements can be made easily with this. T 2 T 1 is the temperature difference before and after heating or cooling K. Given Mass of quinizarin - 06677 g.
There are different ways to determine the specific heat capacity of water. Aquí nos gustaría mostrarte una descripción pero el sitio web que estás mirando no lo permite. The only thing you need to remember is that you have to use consistent units for mass.
Heat Flux DSC comprises the sample and reference holder the heat resistor the heat sink and the heater. The definition of the calorie is based on the specific heat of water defined. In your own words describe the concept of specific heat capacity and the effects that it has on temperature changes.
The mass of the material m The temperature change that occurs DeltaT The specific heat capacity of the material c which you can look up. Mass of octane m 1750g. 12744778 Latent heat capacity of steam.
The energy you just calculated Q water reflects energy released by the total amount of food burned or M f - M i grams of food. In the previous article we discussed the specific heat capacity of substances. This experiment is an extremely quick and relatively precise specific heat capacity test for a solid sample.
To calculate the energy required to raise the temperature of any given substance heres what you require. 11635978 Jkg Homework Equations. One type in widespread use called a bomb calorimeter basically consists of an enclosure in which the reaction takes place surrounded by a liquid such as water that absorbs.
This is the amount of heat required to raise 1 gram of that substance by 1C. Give an example of specific heat capacity that you see in your everyday life. The specific heat also called specific heat capacity is the measure of the heat energy that a substance in a unit quality absorbs or releases when the.
The water increases in temperature by 10 degrees C. A calorimeter is an object used for calorimetry or the process of measuring the heat of chemical reactions or physical changes as well as heat capacityDifferential scanning calorimeters isothermal micro calorimeters titration calorimeters and accelerated rate calorimeters are among the most common types. Heat Flux Type and Power Compensation Type.
Dieter Haemmerich in Principles and Technologies for Electromagnetic Energy Based Therapies 2022. The vessel is filled with water and the fuel is burned leading to the heating of the water. Calorimeters have been designed in great variety.
It is usually expressed as calories per degree in terms of the actual amount of material being considered most commonly a mole the molecular weight in grams. If you have a specific heat capacity in Jg C then you need the mass of the substance in grams. A simple calorimeter just consists of a thermometer attached to a.
This will require 2669 kJ of heat energy. C10H8s was burned in a bomb calorimeter. Find the heat transferred to the calorimeter if the heat capacity of the calorimeter is 893 kJK.
DSC is a commercially available instrument which has two 2 types. M is the mass of a material g. The heat capacity in calories per gram is called specific heat.
Place one litre 1 kg of water in the calorimeter. Calorimeter device for measuring the heat developed during a mechanical electrical or chemical reaction and for calculating the heat capacity of materials.

Constant Volume Calorimetry For More Precise Work Than The Coffee Cup Calorimeter The Heat Capacity Of The Entire C Coffee Cups Chemistry Education Chemistry

Chemistry Thermochemistry 28 Of 37 Combustion In A Bomb Calorimeter Ex 2
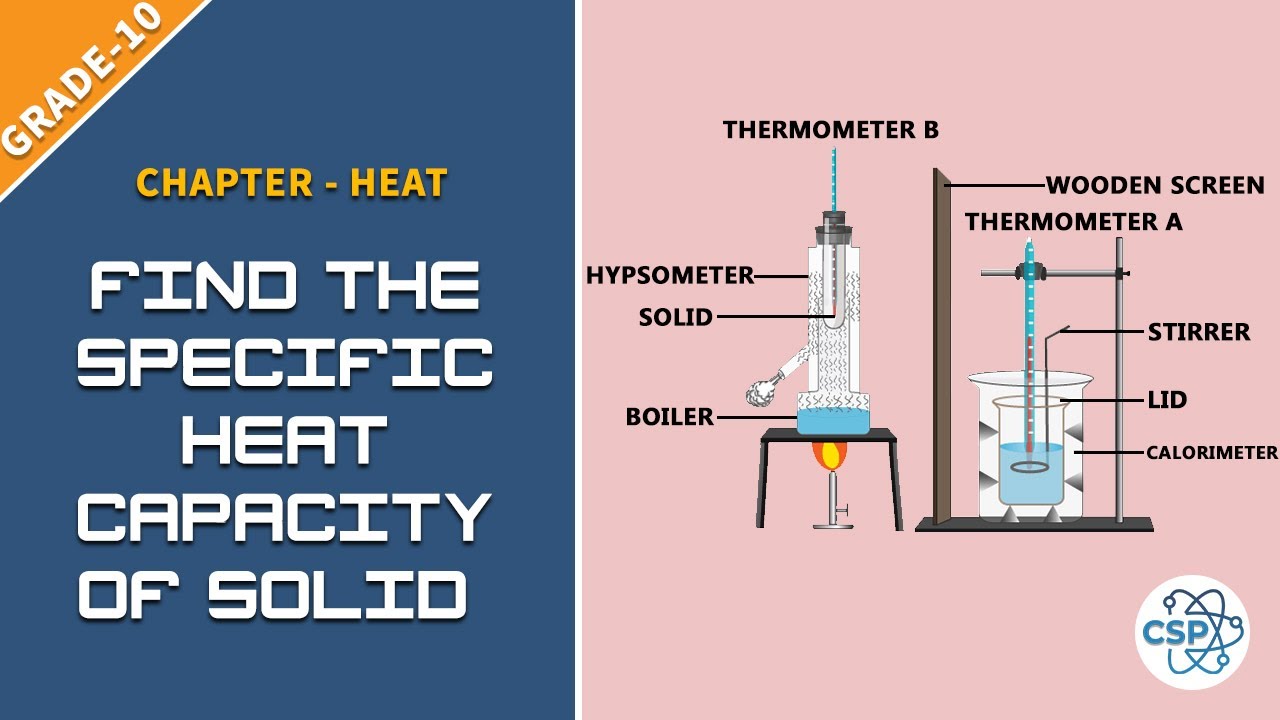
To Find The Specific Heat Capacity Of Solid By Using Method Of Mixtures See Class 10 Physical Properties Heat Science Experiments

Pin By Redacted On Chemistry Education Chemistry Education What Is Science Ap Chem

50 Calorimetry Worksheet Answer Key Chessmuseum Template Library Worksheets Capacity Worksheets Letter Reversals

Specific Heat Worksheet Answers Luxury Heat Transferspecific Heat Problems Worksheet Answers Worksheets Worksheet Template Spelling Words
0 Response to "Heat Capacity of Calorimeter"
Post a Comment